CRISPR-Edited T Cells
High editing efficiency with reliable post-editing functionality. Order Primary T Cells supplied by EditCo or work with our team to edit your primary cell type of interest.
Efficient edits, functional T cells, better science
-
Confident Knockouts: Gene-edited cells guaranteed with Editing Efficiency of ≥80% with our smart multi-guide design
-
Faster Results: EditCo's 7-day editing protocol delivers results in 2 weeks or faster
-
Flexibility: Choose from EditCo-supplied cells or onboard your CD8+ or CD4+ T cells.
-
Quality: High cell viability and editing levels without affecting functionality.
Get Guaranteed Editing Results in Primary Cells
Primary Cell KO Pools are made-to-order knockouts that guarantee ≥80% editing efficiency of Cell Pools in human primary cells generated in 2 weeks or faster, creating cell pools that are functionally similar to a clone. As primary cell clones are biologically difficult to produce, EditCo’s high editing efficiency provides a unique approach to generating cell populations of interest.
Whether you are leveraging CRISPR for validating targets for drug discovery or modeling a biological process for the investigation of a novel gene of interest, failed experiments lead to a loss of time and resources. EditCo’s Primary Cell KO Pools are the solution for obtaining quick and reliable functional data while reducing the risk for false negatives in your critical assays.
Other Primary Cells?
EditCo is expanding its Primary Cell portfolio. Please reach out to inquire about additional cell types, including human primary fibroblasts, monocytes/macrophages, NK cells, B cells and mouse primary cell types.
Focus on your experiment and leave CRISPR to us.
Features
Cell Source
- EditCo supplied
- Customer supplied
Available Edits
- Single knockout
CRISPR Design
- Multi-guide synthetic modified sgRNA (standard)
Deliverables
- Regular updates on your order's progress
- 2 vials of edited cell pools with >1,000,000 cells/vial (pools consist of a heterogeneous population of edited and unedited cells)
- Control-transfected cell pools (2 vials)
- Sequence of synthetic gRNA used
- Primer sequences used for NGS sequencing
- NGS sequencing analysis report for each edited pool after expansion
- Comprehensive QC report that includes the following information: mycoplasma test (positive/negative) and passage number
Edited CD8+ T cell Data
High and Stable Editing Efficiency of CD8+ T cell pools
Video: Edited T Cells are seen killing a target cell via a BiTE antibody-mediated “redirected tumor killing” assay. Bright Field: CD8+ T Cells. Green: CFSE labeled Raji (CD19+) Cells. Red: Dead cells stained with DRAQ7 membrane impermeable viability dye.
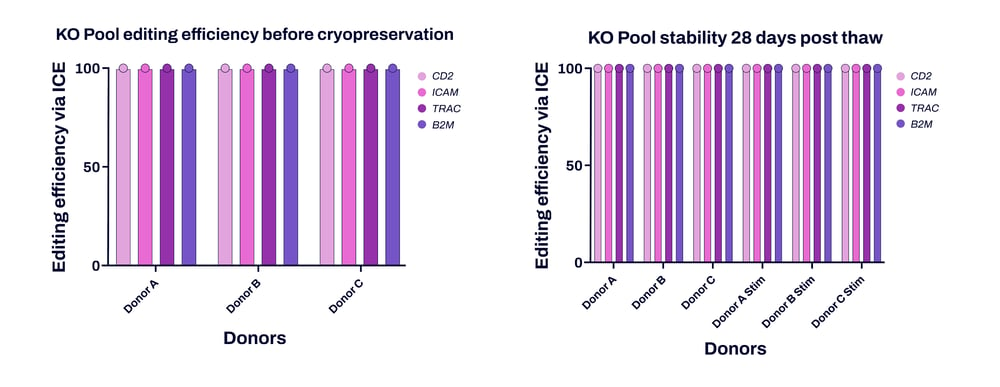
Figure 1. High editing efficiency across multiple donors. Three different donors (A, B and C) show high editing efficiency (>90%), as determined by ICE analysis, across various targeted loci with little change between deliverable (before cryopreservation, left graph) and extended culture (28 days post-thaw, right graph). Cell stimulation after thawing did not affect KO levels.
High viability and expansion of edited pools

Figure 2. EditCo KO CD8+ T cell pools can be thawed and expanded for weeks, with or without TCR stimulation. Edited CD8 T cell pools were thawed and plated in G-Rex™-24 consumables at 1E6 cells per well in Immunocult™ T cell expansion media supplemented with 100ng/ml recombinant human Interleukin-2. Stimulated samples were treated with Immunocult™ CD2-CD3-CD28 T cell activator for 3 days. Cultures were fed every 3 days by replacing 90% volume with fresh media. On Day 14* cell number was returned to 1E6 cells per well to prevent overgrowth.
Biologically Relevant

Figure 3. Preserving cell function. EditCo T cell pools undergo a single round of activation to preserve normal cell function and prevent the cells from acquiring an overstimualted or exhausted phenotype. This is readily seen by the transient expression of activation markers (CD25, CD69) and exhaustion markers (PD1).
Antigen-specific T cell Activation
Figure 4. T cell activation measured by CD107a. Edited CD8+ pools were stimulated with mixed viral peptides (CEF, REF 3) presented on HLA-1 matched B-cells. After 2 rounds of stimulation T cells were incubated with CEF peptides for 4 hours, and cytotoxic activation was measured by CD107a surface localization. WT, CD2, ICAM-2 edited pools showed robust CD107a expression compared to DMSO controls. As expected, B2M and TRAC knockout pools showed no induction above baseline, given their role in antigen presentation and recognition.
Edited CD4+ T cell Data
Stability and Functionality Post-Editing
Figure 1. Editing Efficiency and Viability for CD4+ T cell pools. Values represent the average of three different donors showing high editing efficiency, as determined by ICE analysis, across a variety of loci (blue bars, mean KO scores +- SEM) without affecting viability at the time of freezing (cyan dots).
Figure 2. Editing Efficiency leads to protein depletion as shown by flow cytometry (BD Symphony A1).
High viability and expansion of edited pools
The editing process and further expansion before freezing do not affect cell fitness. Edited cells can be thawed and expanded for any desired downstream assay, maintaining high cell viability and editing levels (Figure 3).
Figure 3. CD4+ pool stability after thaw. Panel A. PD1 expression in TCR activator-treated cultures peaked on day 3 in all tested donors (edited and mock samples). Isotype control staining over the same period is shown for reference. Panel B. Relative fold expansion for edited pools cultured with TCR activator or IL2 alone. Panel C. Pools of 4 different edits from 3 separate donors showed >90% viability and no decrease in editing efficiency after 14 days in culture.
High functionality
Edited CD4+ T cells produced levels of IFNg, IL2 and TNFa after mitogenic stimulation that correlated with known target gene function.
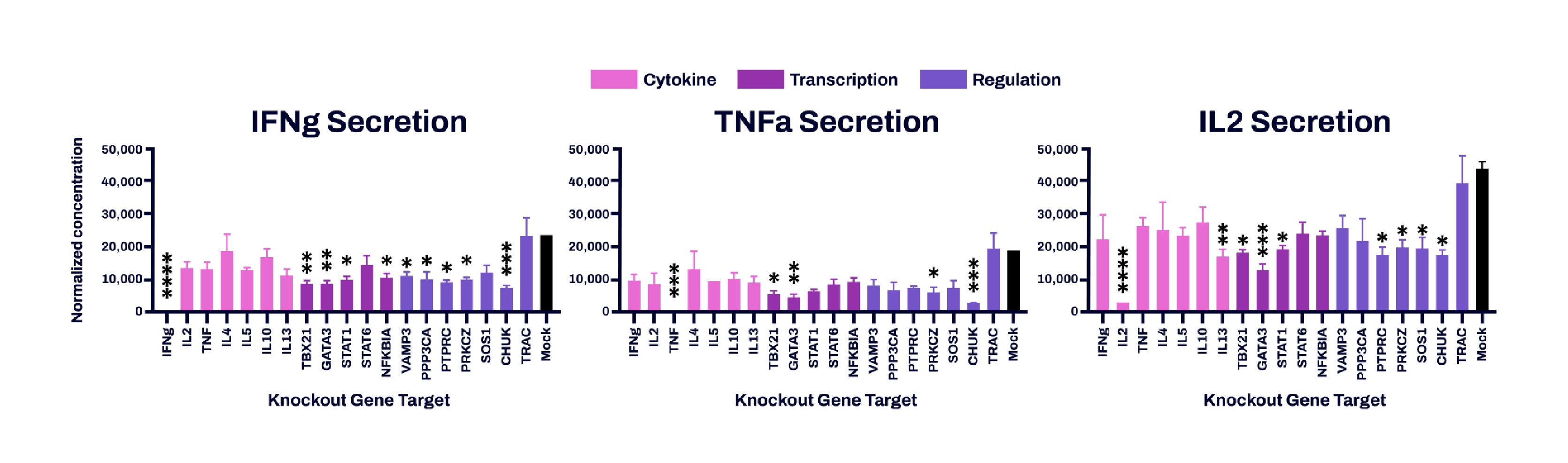
Figure 4. Cytokine secretion in edited cell pools following cell expansion. Edited cells from 3 donors were thawed and then stimulated with PMA/ionomycin for 6 hours. Supernatants were harvested and measured for concentrations of IFNG (left panel), IL2 (center panel) and TNFA (right panel) as well as IL10 and IL5 (not shown) by FACS-ELISA (Miltenyi). Values were quantile normalized to reduce batch effects. Following ANOVA, Tukey’s multiple comparison test was performed between Mock and each gene target to determine statistical significance. (*) p<0.05; (**) p<0.01; (***) p<0.005; (****) p< 0.0001.
Figure 5. Intracellular Cytokine measurement in edited cell pools. Knockout CD4+ T cell pools of IFNg, IL2 or TNFa were thawed and stimulated with PMA/ionomycin for 6 hours. Cells were stained for CD3, CD4 and viability, permeabilized and then stained for presence of IFNG, IL2 or TNFA. Production of either cytokine in CD3/CD4/Live gated events for each Gene Target knockout culture (Bottom histograms) was compared to unedited control (Mock, Middle histograms) as well as TRAC knockout (Top histograms).
Resources
Integrating our core CRISPR expertise, high-quality reagents, and automated processes, we deliver the best edited cell-based models at any scale.

Use cases diam faucibus donec pretium leo risus commodo sit. Phasellus a sit.



Quisque amet eu viverra aliquam sed. Montes nulla nisi sit vel urna leo. Nisi donec consectetur bibendum tortor. Gravida arcu lorem venenatis nulla sodales quis sed.
Testimonials consectitur amet sed
Congue fermentum eros leo commodo. Integer vitae vitae ac amet. Vitae sed lectus vel lacus adipiscing nulla facilisis mauris porttitor. Viverra elementum odio vitae id.

Viverra aliquam volutpat interdum quisque vel mattis. Malesuada aliquam egestas pharetra a tempus ullamcorper egestas.

Nibh luctus pulvinar sagittis faucibus commodo vitae purus. Ullamcorper dictum.

Congue fermentum eros leo commodo. Integer vitae vitae ac amet. Vitae sed lectus vel lacus adipiscing nulla facilisis mauris porttitor. Viverra elementum odio vitae id.

Congue fermentum eros leo commodo. Integer vitae vitae ac amet. Vitae sed lectus vel lacus adipiscing nulla facilisis mauris porttitor. Viverra elementum odio vitae id.

Congue fermentum eros leo commodo. Integer vitae vitae ac amet. Vitae sed lectus vel lacus adipiscing nulla facilisis mauris porttitor. Viverra elementum odio vitae id.

Lectus Pluristyx eu vel leo lectus mi. Viverra cursus neque eget proin.
Pellentesque nec tempus ultrices purus amet adipiscing aliquam. Posuere imperdiet adipiscing non blandit non. Sed eros vel sodales magna sem tincidunt orci risus. Molestie hendrerit cursus faucibus ac neque eget turpis. Arcu leo risus iaculis mi. Tortor bibendum sed cras pretium leo.
0+
0+
0+
Lorem Ipsum Dolor Heading
Etiam sollicitudin, ipsum eu pulvinar rutrum, tellus ipsum laoreet sapien, quis venenatis ante odio sit amet eros. Duis arcu tortor, suscipit eget, imperdiet nec, imperdiet iaculis, ipsum. Maecenas nec odio et ante tincidunt tempus. Donec elit libero, sodales nec, volutpat a, suscipit non, turpis. Quisque id mi.
Resources aliquam procte










