CRISPR-edited Knock-in iPS Cells
Accelerate disease model development, drug discovery, and regenerative medicine with the high editing efficiency and precise genomic integration of EditCo's edited iPS Cells.
Unlock advanced CRISPR knock-ins for neuroscience and regenerative medicine research.
-
Quality: Maintain iPSC quality, pluripotency, and cell integrity with EditCo’s editing process.
-
Precision: Focus on the phenotype of your desired edit and minimize off-target effects with transient transfection.
-
Unlocked Capacity: Experience faster lead times with EditCo’s high-throughput automated platform.
Achieve Precise CRISPR Knock-Ins While Preserving iPSC Quality
Elevate your neuroscience, cardiovascular, or regenerative medicine research with EditCo's precise CRISPR knock-in edits in iPSCs. Let EditCo handle the complex editing process so you can focus on assay development, differentiation, and other downstream applications. Our robust, automated platform ensures high editing efficiencies while maintaining cell quality and pluripotency, providing you with reliable and artifact-free CRISPR-edited iPSCs.
EditCo offers a range of knock-ins, including single nucleotide variants (SNVs), tags, and <100 bp insertions, available in both homozygous and heterozygous states, and in clone or pool formats. Empower your research and accelerate your discoveries with EditCo's advanced CRISPR technology.
Single-guide RNA Knock-in Cell Pools
High Efficiency CRISPR iPSC Knock-ins with DIY Clonal Isolation
EditCo’s Knock-in iPS Cell Pools provide a heterogeneous mix of CRISPR-edited and unedited cells, giving you high editing efficiency without clonal isolation. Benefit from EditCo’s optimized CRISPR platform, achieving precise edits while allowing you the flexibility to perform clonal isolation yourself. For those seeking a fully automated process, explore our Knock-in iPS Cell Clones for guaranteed homogeneity and convenience.
Features
Cell Source
- EditCo supplied (standard)
- Customer supplied
Available Edits
- SNV, Tag, or Insersion
- A heterogeneous population of edited and unedited cells
CRISPR Design
- Synthetic modified sgRNA (standard)
- Donor ssODN (standard)
Add-ons
- QC: Pluripotency testing
Deliverables
- Regular updates on your order's progress
- Edited cell pools (2 vials with 5 million cells/vial)
- Mock-transfected cell pools (2 vials with 5 million cells/vial)
- Sequence of synthetic sgRNA used
- Primer sequences used for NGS sequencing
- NGS sequencing analysis report for each edited pool after expansion.
- Comprehensive QC report that includes the following information: mycoplasma test (positive/negative), passage number, and analysis for add-on QC
Sequencing deliverable note: For large knock-ins and non-human/mouse cell types an alignment between the Sanger sequencing data of the edited pool and the knock-in sequence will be provided
Robust Editing Across Different iPSC Lines
EditCo’s robust, automated editing platform results in high knock-in efficiency across a range of workhorse iPSC lines and patient derived iPS cell lines, ensuring a high success rate in any iPSC line.
Figure 1. Average knock-in editing efficiency by knock-in type. Knock-in efficiencies were all above 31% and performed in a variety of EditCo-supplied and customer supplied iPSC lines. Tags and Small KIs were all less than 100 bps in length. Knock-in edits were performed using RNPs and ssODNs and editing efficiency was assessed in the pool stage before proceeding to clonal isolation. DNA in each population of cells was PCR-amplified around the cut site, Sanger-sequenced, and submitted for ICE analysis to quantify editing efficiency.
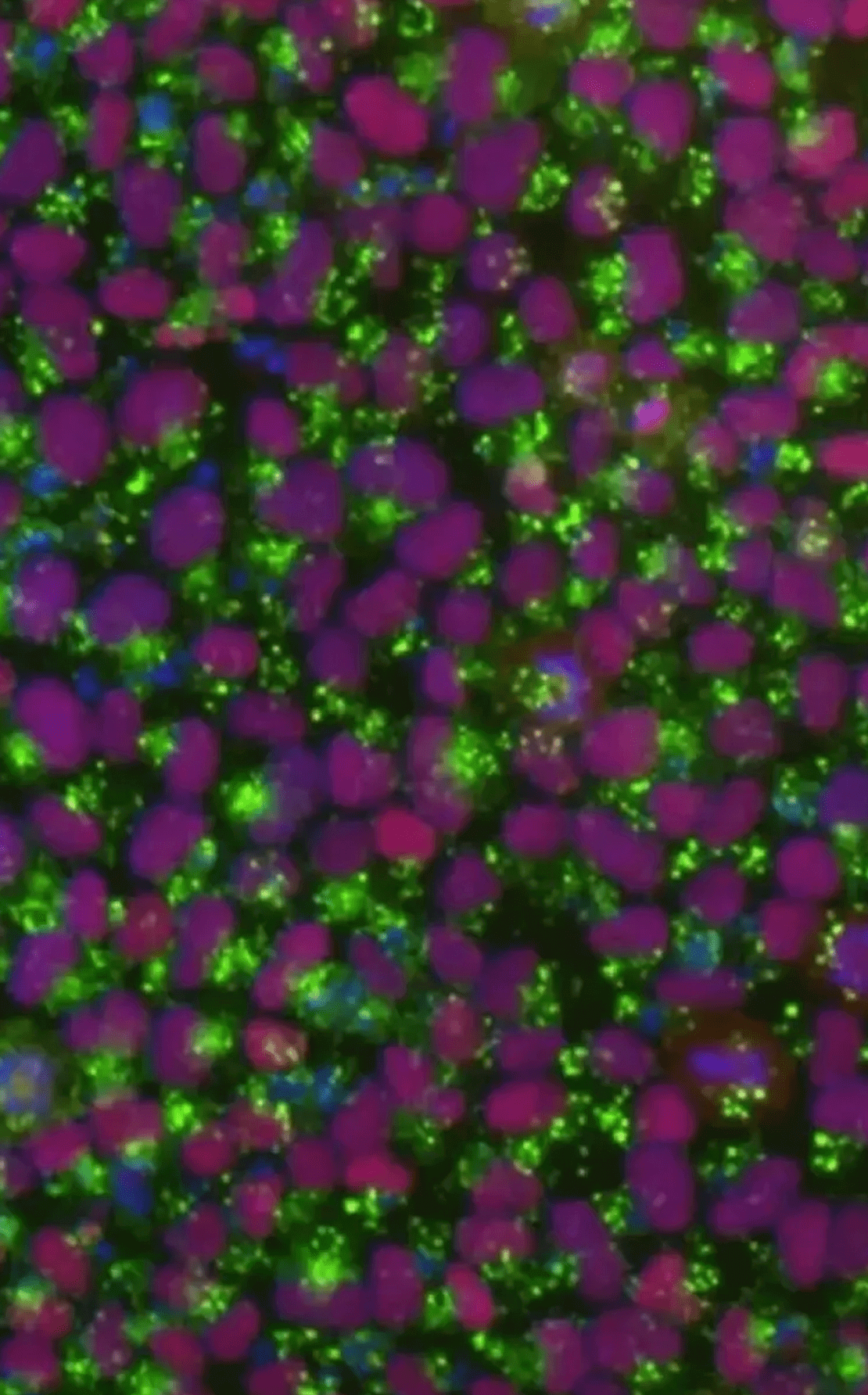
EditCo Delivers Pluripotent Edited Cells
Quality comes first in the development of EditCo’s Knock-in iPS Cells by using our automated workflow and transient RNPs to maintain pluripotency of your cells.

Figure 3. iPS cells were assessed for standard pluripotency markers, three days post-editing.
Single-guide RNA Knock-in Cell Clones
Move Directly Into Your Functional Assays with Confidence
Streamline your research with EditCo’s Knock-in iPS Cell Clones, a homogeneous population of iPSC cells derived from single CRISPR-edited cell. Focus on your functional assays and downstream applications while we handle the entire CRISPR editing and single-cell cloning process, ensuring you receive high-quality, reliable clones to accelerate your scientific breakthroughs.
Features
Cell Source
- EditCo supplied (standard)
- Customer supplied
Genetic Modifications
- SNV, Tag, or Insertion
- Homozygous or Heterozygous Edits
CRISPR Design
- Synthetic modified sgRNA (standard)
- Donor ssODN (Standard)
Add-Ons
- Additional clones
- QC: Pluripotency Testing
- QC: Karyotype Testing
Deliverables
- Regular milestone updates on your order's progress
- 2 independent clones with the required knock-in (2 vials of each clone with 500,000 cells/vial)
- Mock-transfected cell pools (2 vials with 500,000 cells/vial)
- Sequence of synthetic sgRNA and HDR template used
- Primer sequences used for NGS sequencing
- NGS sequencing analysis report for each edited pool after expansion.
- Comprehensive QC report that includes the following information: mycoplasma test (positive/negative), passage number, and analysis for add-on QC
Sequencing deliverable note: For large knock-ins and non-human/mouse cell types an alignment between the Sanger sequencing data of the edited pool and the knock-in sequence will be provided
Precise Single Nucleotide Variants (SNVs)
Figure 3. 100% SNV editing in iPS cell clones. A CRISPR-edited homozygous clone containing a single nucleotide change from cytosine (C, bottom) to thymine (T, top). The SNV change is enclosed by an orange box across both traces. The target sequence (underlined in black) and the PAM site (underlined with a red dashed line) are indicated in the control trace. Human iPS cells were electroporated with sgRNA and Cas9 (as RNPs) along with a ssODN containing the single nucleotide change. To produce clonal populations, single cells were isolated using limiting dilution and expanded.
Use Our iPS Cell Lines or Onboard Your Own
EditCo-supplied cell lines available for all engineered iPS cell orders at no additional cost

* Parental vials available for evaluation prior to booking an edit
Resources
Integrating our core CRISPR expertise, high-quality reagents, and automated processes, we deliver the best edited cell-based models at any scale.

Use cases diam faucibus donec pretium leo risus commodo sit. Phasellus a sit.



Quisque amet eu viverra aliquam sed. Montes nulla nisi sit vel urna leo. Nisi donec consectetur bibendum tortor. Gravida arcu lorem venenatis nulla sodales quis sed.
Testimonials consectitur amet sed
Congue fermentum eros leo commodo. Integer vitae vitae ac amet. Vitae sed lectus vel lacus adipiscing nulla facilisis mauris porttitor. Viverra elementum odio vitae id.

Viverra aliquam volutpat interdum quisque vel mattis. Malesuada aliquam egestas pharetra a tempus ullamcorper egestas.

Nibh luctus pulvinar sagittis faucibus commodo vitae purus. Ullamcorper dictum.

Congue fermentum eros leo commodo. Integer vitae vitae ac amet. Vitae sed lectus vel lacus adipiscing nulla facilisis mauris porttitor. Viverra elementum odio vitae id.

Congue fermentum eros leo commodo. Integer vitae vitae ac amet. Vitae sed lectus vel lacus adipiscing nulla facilisis mauris porttitor. Viverra elementum odio vitae id.

Congue fermentum eros leo commodo. Integer vitae vitae ac amet. Vitae sed lectus vel lacus adipiscing nulla facilisis mauris porttitor. Viverra elementum odio vitae id.

Lectus Pluristyx eu vel leo lectus mi. Viverra cursus neque eget proin.
Pellentesque nec tempus ultrices purus amet adipiscing aliquam. Posuere imperdiet adipiscing non blandit non. Sed eros vel sodales magna sem tincidunt orci risus. Molestie hendrerit cursus faucibus ac neque eget turpis. Arcu leo risus iaculis mi. Tortor bibendum sed cras pretium leo.
0+
0+
0+
Lorem Ipsum Dolor Heading
Etiam sollicitudin, ipsum eu pulvinar rutrum, tellus ipsum laoreet sapien, quis venenatis ante odio sit amet eros. Duis arcu tortor, suscipit eget, imperdiet nec, imperdiet iaculis, ipsum. Maecenas nec odio et ante tincidunt tempus. Donec elit libero, sodales nec, volutpat a, suscipit non, turpis. Quisque id mi.
Resources aliquam procte







